Case Series/Study
(CS-086) A Case Series of ON101 in Acute Radiation Dermatitis
Acute radiation dermatitis (ARD) is a common adverse effect of radiotherapy, particularly in cases where the skin is included in the target volume, such as head and neck cancer or breast cancer. ARD severity ranges from mild erythema to moist desquamation and, in severe cases, necrosis. Current management strategies for mild to moderate ARD typically include interventions such as moist desquamation care, topical corticosteroids, and antibiotics. ON101, a novel topical macrophage regulator, has shown the ability to inhibit the NLRP3 inflammasome cascade and suppress pro-inflammatory cytokines through M1 macrophage modulation in preclinical studies. This case series aimed to assess the clinical efficacy of ON101 in managing ARD.
Methods: Seven patients with grade 3 to grade 4 ARD were treated with ON101 cream, with secondary dressings applied as needed. The primary endpoints were the resolution of erythema and wound healing. Patient-reported outcomes, including treatment satisfaction and quality-of-life improvements, were also documented.
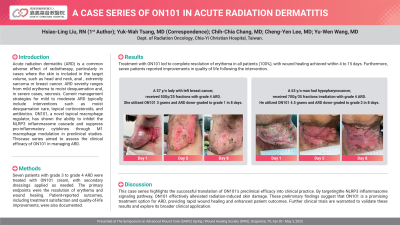
Results: Treatment with ON101 led to complete resolution of erythema in all patients (100%), with wound healing achieved within 4 to 15 days. Furthermore, four patients reported improvements in quality of life following the intervention.
Discussion: This case series highlights the successful translation of ON101’s preclinical efficacy into clinical practice. By targeting the NLRP3 inflammasome signaling pathway, ON101 effectively alleviated radiation-induced skin damage. These preliminary findings suggest that ON101 is a promising treatment option for ARD, providing rapid wound healing and enhanced patient outcomes. Further clinical trials are warranted to validate these results and explore its broader clinical application.

.jpg)