Case Series/Study
(CS-090) Adipose Tissue Allograft as a Novel Adjunct Therapy for Lipodermatosclerosis and Venous Leg Ulcer Care
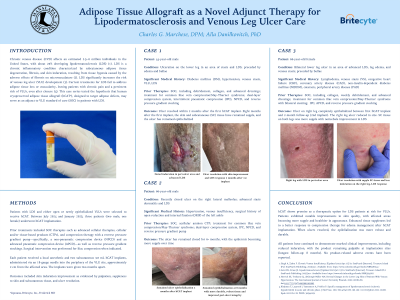
Chronic venous disease (CVD) affects an estimated 2.5–6 million individuals in the United States, with about 20% developing lipodermatosclerosis (LDS). LDS is a chronic inflammatory condition characterized by subcutaneous adipose tissue degeneration, fibrosis, and skin induration, resulting from tissue hypoxia caused by the adverse effects of fibrosis on microvasculature. LDS significantly increases the risk of venous leg ulcer (VLU) development. Current treatments for LDS fail to address adipose tissue loss or avascularity, leaving patients with chronic pain and a persistent risk of VLUs, even after closure. This case series tested the hypothesis that human cryopreserved adipose tissue allograft (hCAT*), designed to target adipose defects, may serve as an adjunct to VLU standard of care (SOC) in patients with LDS.
Methods:
Patients with LDS and either open or newly epithelialized VLUs were selected to receive hCAT. Prior treatments included SOC therapies such as advanced cell therapies and compression therapy with reverse pressure gradient pumps and devices. Each patient received two 3.0 mL subcutaneous hCAT implants, administered via an 18-gauge needle into the periphery of the VLU site, two months apart. Outcomes included skin induration improvement by palpation and ulcer resolution.
Results:
Between July and August 2024, two male patients, aged 44 and 70, each received two hCAT implantations. Both had a history of chronic VLUs with underlying venous stasis and significant LDS. At 4-months follow-up, both patients demonstrated significant improvements in the clinical appearance and induration at the implantation sites. Notably, the quality of the skin in previously affected areas improved markedly, becoming more supple, with a healthier overall appearance. hCAT remained palpable at implantation sites, and no product-related adverse events were observed.
Discussion:
hCAT shows promise as a therapeutic option for LDS patients at risk for VLUs. Initial outcomes suggest safety, durability, and clinical improvement in the implanted areas. Long-term follow-up is underway to assess sustained efficacy and safety.

.jpg)