Laboratory Research
(LR-025) Comparative Analysis of Xenograft ECM Particulates for Wound Management Applications
Friday, May 2, 2025
7:45 PM - 8:45 PM East Coast USA Time
Sarah Moreno, MS – MIMEDX Group, Inc.; Michelle Massee, MS; John Harper, PhD
Introduction: During wound healing, the extracellular matrix (ECM) is rebuilt to provide a structural framework allowing for cell migration and proliferation to repair the defect. When necessary, an advanced biomaterial may be used to supplement or expedite this process. Naturally-derived scaffolds from animal tissues rely on the inherent composition and organization of the ECM which is largely comprised of macromolecules such as collagen. Collagen-based scaffolds not only trigger essential processes such as cell adhesion, migration, chemotaxis, and tissue development but also offer properties that facilitate the infiltration of cells while being biocompatible, biodegradable, and non-toxic.1 This study compared the in vivo biocompatibility of xenograft ECM scaffolds from bovine*, porcine#, and piscine$ sources.
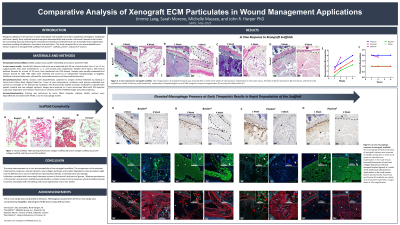
Methods: Hematoxylin and eosin (H&E) staining was used to visualize the structure of each ECM particulate. The in vivo response was evaluated after subcutaneous implantation of each ECM particulate in athymic nude mice, followed by histological and immunofluorescent assessment of the implant sites at 1, 2, and 4 weeks post-implantation. H&E staining was used for the histopathological assessment to evaluate parameters associated with biocompatibility. Collagen deposition was demonstrated through immunofluorescence analysis.
Results: Histological analysis shows the particulate structure and collagen type I composition with the exception of the piscine product for which a commercially available COL I antibody was unavailable. Following subcutaneous implantation of each ECM particulate, the piscine ECM particulate showed evidence of inflammation associated with early tissue degradation. The bovine and porcine ECM scaffolds elicited minimal inflammation and exhibited matrix remodeling associated with cell infiltration over time. Neovascularization was evident with all ECM scaffolds. Collagen deposition was present at each time point for all products.
Discussion: The study demonstrates the in vivo biocompatibility of xenograft ECM scaffolds. The source material impacted the inflammatory response and rate of tissue degradation. This study highlights the ability of ECM scaffolds to support wound management, particularly in deep wounds where the scaffold can conform to the surface of the wound.
Methods: Hematoxylin and eosin (H&E) staining was used to visualize the structure of each ECM particulate. The in vivo response was evaluated after subcutaneous implantation of each ECM particulate in athymic nude mice, followed by histological and immunofluorescent assessment of the implant sites at 1, 2, and 4 weeks post-implantation. H&E staining was used for the histopathological assessment to evaluate parameters associated with biocompatibility. Collagen deposition was demonstrated through immunofluorescence analysis.
Results: Histological analysis shows the particulate structure and collagen type I composition with the exception of the piscine product for which a commercially available COL I antibody was unavailable. Following subcutaneous implantation of each ECM particulate, the piscine ECM particulate showed evidence of inflammation associated with early tissue degradation. The bovine and porcine ECM scaffolds elicited minimal inflammation and exhibited matrix remodeling associated with cell infiltration over time. Neovascularization was evident with all ECM scaffolds. Collagen deposition was present at each time point for all products.
Discussion: The study demonstrates the in vivo biocompatibility of xenograft ECM scaffolds. The source material impacted the inflammatory response and rate of tissue degradation. This study highlights the ability of ECM scaffolds to support wound management, particularly in deep wounds where the scaffold can conform to the surface of the wound.

.jpg)