Laboratory Research
(LR-039) Human Keratin Matrices Modulate Inflammatory Crosstalk Between Keratinocytes and Macrophages
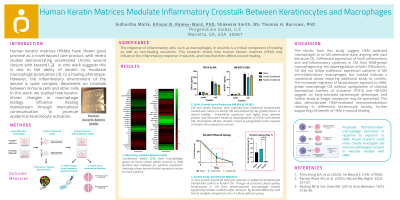
Keratin biomaterials such as the human keratin matrix (HKM) have shown great promise as a novel wound care product, with recent in vivo and clinical studies demonstrating accelerated chronic wound closure[1,2]. In vitro work suggests this is due in part to the ability of keratin to modulate the inflammatory environment[2], specifically macrophage polarization[3], to a healing phenotype. However, the inflammatory environment of the wound is quite complex, dependent on crosstalk between immune cells and other cells such as keratinocytes. Here, we studied how keratin-driven changes in macrophage biology influence healing downstream through intercellular communication to promote epidermal keratinocyte activation.
Methods: 264.7 RAW macrophages were grown on HKM-coated or control tissue culture surfaces for 3 days, and conditioned media (CM) was collected and analyzed for 63 targets using a cytokine microarray. Primary epidermal keratinocytes were grown in CM for 3 days, after which cell and media fractions were probed for markers of keratinocyte activation (TGF-β and heparin-binding epidermal growth factor (HB-EGF)) by ELISA. A scratch assay was also performed keratinocytes grown in CM or control media, and scratch closure was measured.
Results: Analysis of CM from HKM- and control-grown macrophages showed differential expression of multiple cytokines. Keratinocytes grown in CM from HKM-grown macrophages showed significantly (p< 0.05) reduced scratch sizes compared to those grown in control media at the same timepoints. However, no statistically significantly differences were detected in keratinocyte TGF-β and HB-EGF expression due to CM, though intracellular fractions showed elevated levels of both proteins compared to media.
Discussion:
These results suggest HKM polarized macrophages to an M2-alternative state, aligning with past literature[3]. Differential expression of both inflammatory and anti-inflammatory cytokines in CM from HKM-grown macrophages (e.g. the downregulation of both TNFα and IL-10) did not follow traditional expression patterns of M2 anti-inflammatory macrophages, but instead indicate a transitional phase. Increased migration of keratinocytes exposed to HKM-grown macrophage CM without upregulation of TGF-β and HB-EGF suggest an early-activated keratinocyte phenotype, and further study at longer timepoints may be warranted. This data demonstrates HKM-mediated immunomodulation resulting in differential keratinocyte activity, further supporting the benefit of HKM in wound healing.

.jpg)
