Case Series/Study
(CS-030) Clinical Evaluation of Tendon Repair with the Application of Lyophilized Full Thickness Human Amniotic Membrane
Friday, May 2, 2025
7:45 PM - 8:45 PM East Coast USA Time
Hyun Ju Lim, PhD – Director, Research and Development, Research and Development, LifeLink Foundation; Meghan Richline, DPM – PGY-3, LECOM College of Podiatric Medicine
Introduction: Adhesion formation between repaired tendons and surrounding tissues is one of the main complications after tendon repair resulting in poor clinical outcome. Human amniotic membrane is an established modality in epithelial defects which acts as an anti-inflammatory, nonimmunogenic, and mechanical barrier to promote epithelialization, inhibit fibrosis and scar formation. This case study presents the clinical outcome of two patients who received a lyophilized full thickness human amniotic membrane (FT-AC) after tendon repair surgery as an adhesion barrier.
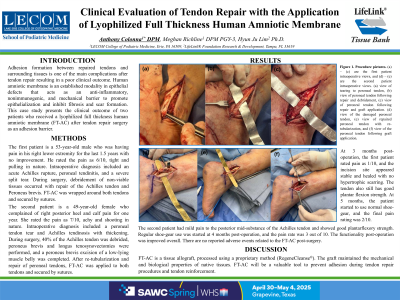
Methods: The first patient is a 53-year-old male who was having pain in his right lower extremity for the last 1.5 years with no improvement. He rated the pain as 6/10, tight and pulling in nature. Intraoperative diagnosis included acute Achilles rupture, peroneal tendinitis, and a severe split tear. During surgery, debridement of non-viable tissues occurred with repair of the Achilles tendon and Peroneus brevis. FT-AC was wrapped around both tendons and secured by sutures.
The second patient is a 49-year-old female who complained of right posterior heel and calf pain for one year. She rated the pain as 7/10, achy and shooting in nature. Intraoperative diagnosis included peroneal tendon tear, and Achilles tendinosis with thickening. During surgery, 40% of the Achilles tendon was debrided, peroneus brevis and longus tenosynovectomy, and peroneus brevis excision of a low-lying muscle belly were completed. Re-tubularization and repair of peroneal tendons, FT-AC was applied and sutured to both tendons.
Results: The pain score in both patients significantly decreased to 1-3/10, and range of motion using repaired tendons improved within 3-4 weeks. There are no reported adverse events related to the FT-AC through 4 months post-surgery. Long-term follow-up is ongoing.
Discussion: FT-AC is a tissue allograft, processed using the proprietary method by LifeLink. The graft maintained the mechanical and biological properties of native tissues. FT-AC will be a valuable tool to prevent adhesion during tendon repair procedures and tendon reinforcement.
Methods: The first patient is a 53-year-old male who was having pain in his right lower extremity for the last 1.5 years with no improvement. He rated the pain as 6/10, tight and pulling in nature. Intraoperative diagnosis included acute Achilles rupture, peroneal tendinitis, and a severe split tear. During surgery, debridement of non-viable tissues occurred with repair of the Achilles tendon and Peroneus brevis. FT-AC was wrapped around both tendons and secured by sutures.
The second patient is a 49-year-old female who complained of right posterior heel and calf pain for one year. She rated the pain as 7/10, achy and shooting in nature. Intraoperative diagnosis included peroneal tendon tear, and Achilles tendinosis with thickening. During surgery, 40% of the Achilles tendon was debrided, peroneus brevis and longus tenosynovectomy, and peroneus brevis excision of a low-lying muscle belly were completed. Re-tubularization and repair of peroneal tendons, FT-AC was applied and sutured to both tendons.
Results: The pain score in both patients significantly decreased to 1-3/10, and range of motion using repaired tendons improved within 3-4 weeks. There are no reported adverse events related to the FT-AC through 4 months post-surgery. Long-term follow-up is ongoing.
Discussion: FT-AC is a tissue allograft, processed using the proprietary method by LifeLink. The graft maintained the mechanical and biological properties of native tissues. FT-AC will be a valuable tool to prevent adhesion during tendon repair procedures and tendon reinforcement.

.jpg)