Clinical Research
(CR-001) Omadacycline for Skin and Soft Tissue Infections: A Multicenter Retrospective Analysis of Efficacy and Safety in Real-world Clinical Practice
Skin and soft tissue infections (SSTIs) present significant challenges in clinical practice, especially due to the growing issue of antibiotic resistance. Omadacycline a first-in-class aminomethylcycline, provides broad-spectrum activity and is available in both oral and intravenous formulations. This study aims to assess the real-world effectiveness and safety of omadacycline in treating SSTIs.
Methods:
This study was a multicenter, retrospective, observational, descriptive analysis of patients treated with omadacycline between July 2019 and December 2023. Patients aged 18 years and older were included if they received omadacycline for at least 48 hours to treat a SSTI. The primary outcome measured was clinical cure, defined as the absence of any signs or symptoms of infection during treatment or within 14 days after discontinuation of omadacycline. The secondary outcome assessed was the incidence of adverse effects.
Results:
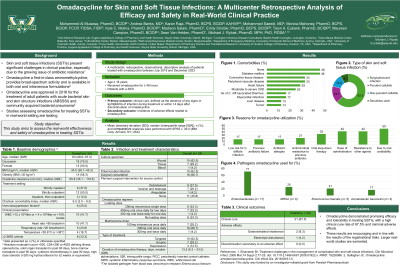
A total of 24 cases from 7 medical centers in the U.S. met the inclusion criteria. The median age of patients was 63 years (IQR: 48.0 – 70.3), with half the cases being female (50.0%) and predominantly Caucasian (72.7%). The most common comorbidities included diabetes mellitus (45.8%), peripheral vascular disease (33.3%), and connective tissue disease (33.3%), with a median Charlson Comorbidity Index of 6.5 (IQR: 2.0 – 9.3). Surgical or wound infections were the most common type of SSTIs, accounting for 62.5% of cases, followed by purulent cellulitis at 16.7%. The primary targeted pathogens included Enterobacterales (70.8%), Enterococcus
species (37.5%), and Methicillin-resistant Staphylococcus aureus (25.0%). The main reasons for utilizing omadacycline were its oral availability (50.0%), ease of administration (41.7%), resistance to other agents (41.7%), and oral step-down therapy (33.3%). Clinical cure was achieved in 87.5% of the cases. Three patients experienced side effects: gastrointestinal intolerance (n=2) and electrolyte disturbances (n=1).
Discussion: Omadacycline demonstrated promising efficacy and tolerability in treating SSTIs, with a high clinical cure rate of 87.5% and minimal adverse effects. These results are encouraging and in line with the results of the registrational trials. Larger real-world studies are warranted.

.jpg)