Case Series/Study
(CS-061) Integrating Multispectral Near-infrared Spectroscopy and Thermography Imaging in the Management of Diabetic Foot Ulcers and Venous Leg Ulcers with Skin Substitutes
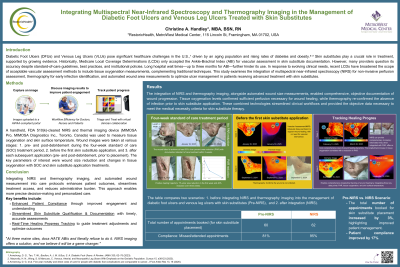
Methods:
A handheld, FDA 510(k)-cleared NIRS and thermal imaging device* was used to measure tissue oxygenation and skin surface temperature. Wound images were taken at various stages: pre- and post-debridement during the four-week standard of care (SOC) treatment period, before the first skin substitute application, and after each subsequent application (pre- and post-debridement, prior to placement). The case series involved four patients, and the key parameters of interest were wound size reduction and changes in tissue oxygenation with SOC and skin substitute application treatments.
Results: The integration of NIRS and thermography imaging, alongside wound size measurements, enabled comprehensive, objective documentation of wound progression. Tissue oxygenation levels confirmed sufficient perfusion necessary for wound healing, while thermography re-confirmed the absence of infection prior to skin substitute application. These combined technologies streamlined clinical workflows and provided the objective data necessary to meet the medical necessity criteria for skin substitute therapy.
Discussion: Non-invasive measurements of tissue oxygenation, in conjunction with skin surface temperature assessment, provide real-time insights that help predict treatment outcomes, guide clinical decision-making, and ensure timely access to advanced therapies like skin substitutes. These technologies enhance clinical workflows by simplifying documentation for insurance coverage and reimbursement. Ultimately, the integration of NIRS imaging into wound care protocols not only improves patient outcomes but also expedites treatment access and reduces the administrative burden on healthcare providers.

.jpg)