Case Series/Study
(CS-041) Efficacy of a Transforming Powder Dressing for Deep and Tunneling Wounds
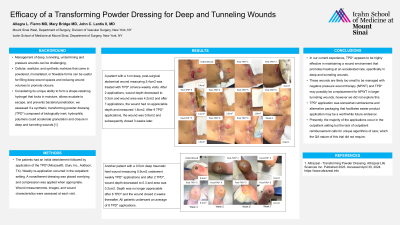
Management of deep, tunneling, undermining and pressure wounds can be challenging. Cellular, acellular, and synthetic matrices that come in powdered, morcellated, or flowable forms can be useful for filling deep wound spaces and reducing wound volumes to promote closure. Considering its unique ability to form a shape-retaining hydrogel that locks in moisture, allows exudate to escape, and prevents bacterial penetration, we assessed if a synthetic, transforming powder dressing (TPD*) composed of biologically inert, hydrophilic polymers could accelerate granulation and closure in deep and tunneling wounds.[1]
Methods:
The patients had an initial debridement followed by application of the TPD*(Altrazeal®, Uluru Inc., Addison, TX). Weekly re-application occurred in the outpatient setting. A nonadherent dressing was placed overlying and compression was applied when appropriate. Wound measurements, images, and wound characteristics were assessed at each visit.
Results:
A patient with a 1cm deep, post-surgical abdominal wound measuring 5.4cm2 was treated with TPD* at twice weekly visits. After 2 applications, wound depth decreased to 0.3cm and wound area was 4.2cm2 and after 7 applications, the wound had no appreciable depth and measured 1.6cm2. After 9 TPD* applications, the wound was 0.9cm2 and subsequently closed 3 weeks later. Another patient with a 0.5cm deep traumatic heel wound measuring 0.9cm2 underwent weekly TPD* applications and after 2 TPD*, wound depth decreased to 0.3 and area was 0.2cm2. Depth was no longer appreciable after 6 TPD* and the wound closed 2 weeks thereafter. All patients underwent an average of 8 TPD* applications.
Discussion:
In our current experience, TPD* appears to be highly effective in maintaining a wound environment that promotes healing at an accelerated rate, specifically in deep and tunneling wounds. These wounds are likely too small to be managed with negative pressure wound therapy (NPWT) and TPD* may possibly be a replacememt for NPWT in larger tunneling wounds, however we did not explore this. TPD* application was somewhat cumbersome and alternative packaging that facilitates easier product application may be a worthwhile future endeavor. Presently, the majority of the applications occur in the outpatient setting but the lack of outpatient reimbursement calls for unique algorithms of care; which the QA nature of this trial did not require.

.jpg)