Laboratory Research
(LR-004) Elucidating the Role of sfrp2 in Modulating Organ Fibrosis
Friday, May 2, 2025
7:45 PM - 8:45 PM East Coast USA Time
Kim Cooney, Kim Cooney, PhD – Biological Sciences; Sarika Saraswati, Sarika Saraswati, PhD – Primary Investigator, Biological Sciences
Introduction: Fibrosis is the typical response to injury, leading to distorted architecture, pathologic signaling and organ dysfunction. Secreted frizzled-related protein (sFRP2) is a mesenchyme derived factor that partially down-regulates fibrosis. Yet, the molecular mechanism that regulates sFRP2’s effect on fibroblasts in modulating tissue fibrosis is incompletely understood. We have generated a transgenic mouse model to temporally and spatially regulate the expression of sFRP2 in injury-induced activated fibroblasts. In two different injury models, cardiac (myocardial infarction; MI) and kidney (unilateral ureteral obstruction; UUO), sFRP2 induction exerted an anti-fibrotic effect in sFRP2-overexpressing mice compared to control Cre mice. Based on our preliminary observation, we hypothesize that sFRP2 inhibits fibrosis by modulating the pro-fibrotic signaling pathways in post-injury activated myofibroblasts. To test this hypothesis, we analyzed the pro-fibrotic pathways activated in response to injury in sFRP2 and Cre mice at multiple time points following MI and UUO injuries.
Methods: MI induction following left anterior descending artery (LAD) ligation and UUO was performed by the ligation one ureter in sFRP2 and Cre mice. The animals were fed tamoxifen for five consecutive days prior to injury. Following injury, mice were sacrificed at different time points to assess the spatial-temporal activation of sFRP2 and its effect on modulating fibrosis and associated pathways. Tissue samples were collected for RT-PCR, Western blot and Immunofluorescence analysis.
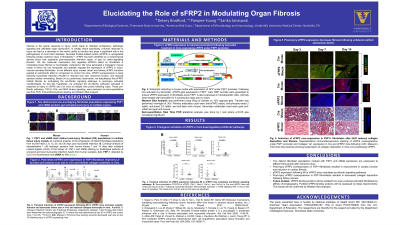
Results: sFRP2 expression was induced post-infarct following tamoxifen treatment in mice expressing sFRP2 under the FSP1 promoter. Transient induction of sFRP2 expression following MI in sFRP2 mice inhibited fibrosis and collagen 1a1 protein expression. Moreover, Masson’s trichrome blue staining of UUO injured kidneys show suppression of collagen deposition in sFRP2 mice compared to Cre mice. Temporal inhibition of TGF-β signaling pathways was also observed in MI and UUO models following sFRP2 over-expression. Future work will focus on elucidating the molecular mechanisms that regulate sFRP2 mediated organ fibrosis. This work will allow us to use regulators, like sFRP2, to target myofibroblast specific molecular pathways during fibrosis, inhibiting pathological fibrosis without affecting repair.
Discussion: Post-injury sFRP2 over-expression in mouse FSP1-fibroblasts resulted in a reduction in fibrosis in two different organ injury models. Elucidating the mechanism that modulates sFRP2’s antifibrotic role will provide valuable insights on how this protein targets fibrosis and promotes regenerative repair. Identification of sFRP2’s role will also provide potential uses for regulators as a way to target specific post-injury fibrotic processes, such as Wnt and TGF-β signaling, and inhibit pathological fibrosis without affecting normal wound healing.
Methods: MI induction following left anterior descending artery (LAD) ligation and UUO was performed by the ligation one ureter in sFRP2 and Cre mice. The animals were fed tamoxifen for five consecutive days prior to injury. Following injury, mice were sacrificed at different time points to assess the spatial-temporal activation of sFRP2 and its effect on modulating fibrosis and associated pathways. Tissue samples were collected for RT-PCR, Western blot and Immunofluorescence analysis.
Results: sFRP2 expression was induced post-infarct following tamoxifen treatment in mice expressing sFRP2 under the FSP1 promoter. Transient induction of sFRP2 expression following MI in sFRP2 mice inhibited fibrosis and collagen 1a1 protein expression. Moreover, Masson’s trichrome blue staining of UUO injured kidneys show suppression of collagen deposition in sFRP2 mice compared to Cre mice. Temporal inhibition of TGF-β signaling pathways was also observed in MI and UUO models following sFRP2 over-expression. Future work will focus on elucidating the molecular mechanisms that regulate sFRP2 mediated organ fibrosis. This work will allow us to use regulators, like sFRP2, to target myofibroblast specific molecular pathways during fibrosis, inhibiting pathological fibrosis without affecting repair.
Discussion: Post-injury sFRP2 over-expression in mouse FSP1-fibroblasts resulted in a reduction in fibrosis in two different organ injury models. Elucidating the mechanism that modulates sFRP2’s antifibrotic role will provide valuable insights on how this protein targets fibrosis and promotes regenerative repair. Identification of sFRP2’s role will also provide potential uses for regulators as a way to target specific post-injury fibrotic processes, such as Wnt and TGF-β signaling, and inhibit pathological fibrosis without affecting normal wound healing.

.jpg)