Case Series/Study
(CS-081) Cell-free Human Amniotic Fluid (cfAF) in the Treatment of Chronic Venous Leg Ulcer in a Patient with Type 2 Diabetes and Chronic Venous Insufficiency: A Case Study
Friday, May 2, 2025
7:45 PM – 8:45 PM East Coast USA Time
Sean O'Connell, PhD – Clinical Science Advisor, Scientific & Medical Affairs, Merakris Therapeutics; Jovan Markovic, MD – General Surgeon, Surgery, Duke University
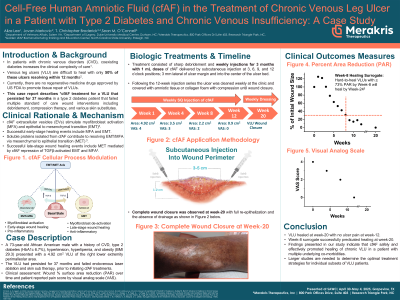
Introduction: Approximately 67% of patients with diabetes mellitus have deep venous incompetence lower legs. Treatment of chronic non-healing venous leg ulcers (VLU) represents a significant health care problem resulting in a reduction of quality of life and significant economic burden due to increased direct healthcare costs and decreased productivity. Non- or poorly-treated VLUs carry a high risk of infection and amputation, especially with concomitant diabetes and/or ischemia. Absence of successful management of non-healing VLUs represents a significant gap in current ulcer management and mandates use of novel safe and effective treatments. This study presents successful treatment of a complex VLU refractory to standard of care for over three years.
Methods: A 73-year-old African American male with a history of chronic venous insufficiency (previously treated with venous laser ablation and skin substitutes), type 2 diabetes, hypertension, hyperlipemia, and obesity (BMI 29.3) presented with a 4.92 cm2 medial peri-malleolar non-healing VLU of the right leg. The VLU had persisted for approximately 37 months prior to presentation. The patient was treated weekly for three months with 1 mL doses (12 treatments) of cfAF* delivered by subcutaneous injection at 3, 6, 9, and 12 o’clock positions; 3 mm outside of the ulcer margin and a single injection into the center of the ulcer bed. Additional procedures consisted of mechanical debridement, visual inspection, ulcer area measurement to determine changes over time and patient reported pain score by visual analog scale (VAS).
Results: During the 12-week cfAF treatment, VLU area decreased from 4.92 cm2 to 0.9 cm2 (- 81.7%). Complete closure, without drainage, was documented two months after the last treatment. No side effects were observed, and the treatment appeared well tolerated. The VAS score decreased from 4 at Baseline to 0 at Week 12, while Dermatology Life Quality Index decreased from 3 at Baseline to 1.
Discussion: The findings presented indicate that cfAF effectively treats chronic non-healing VLUs in complex a patient with multiple co-morbidities. While larger studies are needed to determine optimal application for individual subsets of VLUs patients, initial data from our study strongly suggest that cfAF can be safe and effective in management of complex VLUs.
Methods: A 73-year-old African American male with a history of chronic venous insufficiency (previously treated with venous laser ablation and skin substitutes), type 2 diabetes, hypertension, hyperlipemia, and obesity (BMI 29.3) presented with a 4.92 cm2 medial peri-malleolar non-healing VLU of the right leg. The VLU had persisted for approximately 37 months prior to presentation. The patient was treated weekly for three months with 1 mL doses (12 treatments) of cfAF* delivered by subcutaneous injection at 3, 6, 9, and 12 o’clock positions; 3 mm outside of the ulcer margin and a single injection into the center of the ulcer bed. Additional procedures consisted of mechanical debridement, visual inspection, ulcer area measurement to determine changes over time and patient reported pain score by visual analog scale (VAS).
Results: During the 12-week cfAF treatment, VLU area decreased from 4.92 cm2 to 0.9 cm2 (- 81.7%). Complete closure, without drainage, was documented two months after the last treatment. No side effects were observed, and the treatment appeared well tolerated. The VAS score decreased from 4 at Baseline to 0 at Week 12, while Dermatology Life Quality Index decreased from 3 at Baseline to 1.
Discussion: The findings presented indicate that cfAF effectively treats chronic non-healing VLUs in complex a patient with multiple co-morbidities. While larger studies are needed to determine optimal application for individual subsets of VLUs patients, initial data from our study strongly suggest that cfAF can be safe and effective in management of complex VLUs.

.jpg)